Make your own water-wash flux
2019-05-29 SDIY chemistry science!
The current trends in electronics are to smaller and smaller surface-mount components; lead-free solder alloys and a diversity of surface platings; higher temperatures which mean soldering must be faster to avoid component damage; and avoiding toxic or environmentally-damaging cleaning solvents. All these trends make traditional solder fluxes, like the one in my last article, less effective or appealing. In this article, let's look at water-wash fluxes, which address some of the issues of the older style.
Water-wash fluxes are often called "organic" fluxes, but that's a terrible name for them because although literally true, it has false implications to trap the unwary. These fluxes are "organic" in the sense that they are based on carbon-based chemicals, but all the fluxes traditionally used in electronics were always organic in that sense. Rosin is, after all, a product that comes from living things. Solder fluxes based on inorganic chemicals like ammonium chloride also exist and are used in mechanical applications like plumbing, but inorganic fluxes have never been very popular in electronics because of their corrosiveness. Water-washable fluxes might even be said to be less organic than rosin because they don't require organic solvents for washing. Inorganic acids often are soluble in water too. And there may be a tendency for listeners to confuse "organic" in the sense of "organic chemicals" (which is what applies to water-wash solder fluxes) with "organic" in the sense of "organic foods" (which is irrelevant here). All in all, "water-wash" is a better term for these fluxes because it captures what actually makes them different from others.
As is typical for water-wash fluxes, the flux I'm describing here is very much more active than traditional rosin fluxes, even when they contain activators. It'll be interesting to see how it performs in testing, but my expectation is that it will be the easiest to work with of my formulations, making the solder extremely wet and sticky. The other side of highly active flux is that it's corrosive: the mechanism of action of this flux is more or less that it corrodes the metal surfaces during the soldering process, and it will continue to do so even at room temperature, so it has to be removed soon after use. The dark side of being able to wash flux with water is that, for fluxes of this type that are typically described as "water-wash," you must wash them with water. There are such things as no-clean fluxes that are water-soluble too; I'll discuss one of those in my next article.
A side issue is that water-soluble fluxes often do not dissolve well in non-water solvents used for cleaning electronics, so even if you use one of those other solvents for other reasons, you end up being forced to include water in your cleaning process too. And not all electronic components can withstand washing with water. In a project like the Leapfrog VCF, which includes many expensive high-quality trimmers and panel potentiometers, I do as much cleaning as possible before mounting those, and then have to do the final cleaning steps very carefully with hand brushing instead of immersion, to avoid getting cleaning solvent into the non-washable components. Some things, like stranded wire, may not be damaged by water washing but cannot really be cleaned effectively (there will always be some flux residue trapped between the strands) and those mandate a non-corrosive flux.
But water-wash fluxes have become popular in places where they can be used, even to the point of some hobbyists using both water-wash and no-clean in different parts of the same project, because their advantages for convenience and ease of working are hard to deny. There may also be cost and environmental advantages in avoiding the use of organic solvents for washing; safely disposing of contaminated wash water is usually easier.
The most common activating ingredient in water-wash flux is succinic acid, which is a simple two-carbon backbone with two organic acid groups attached.
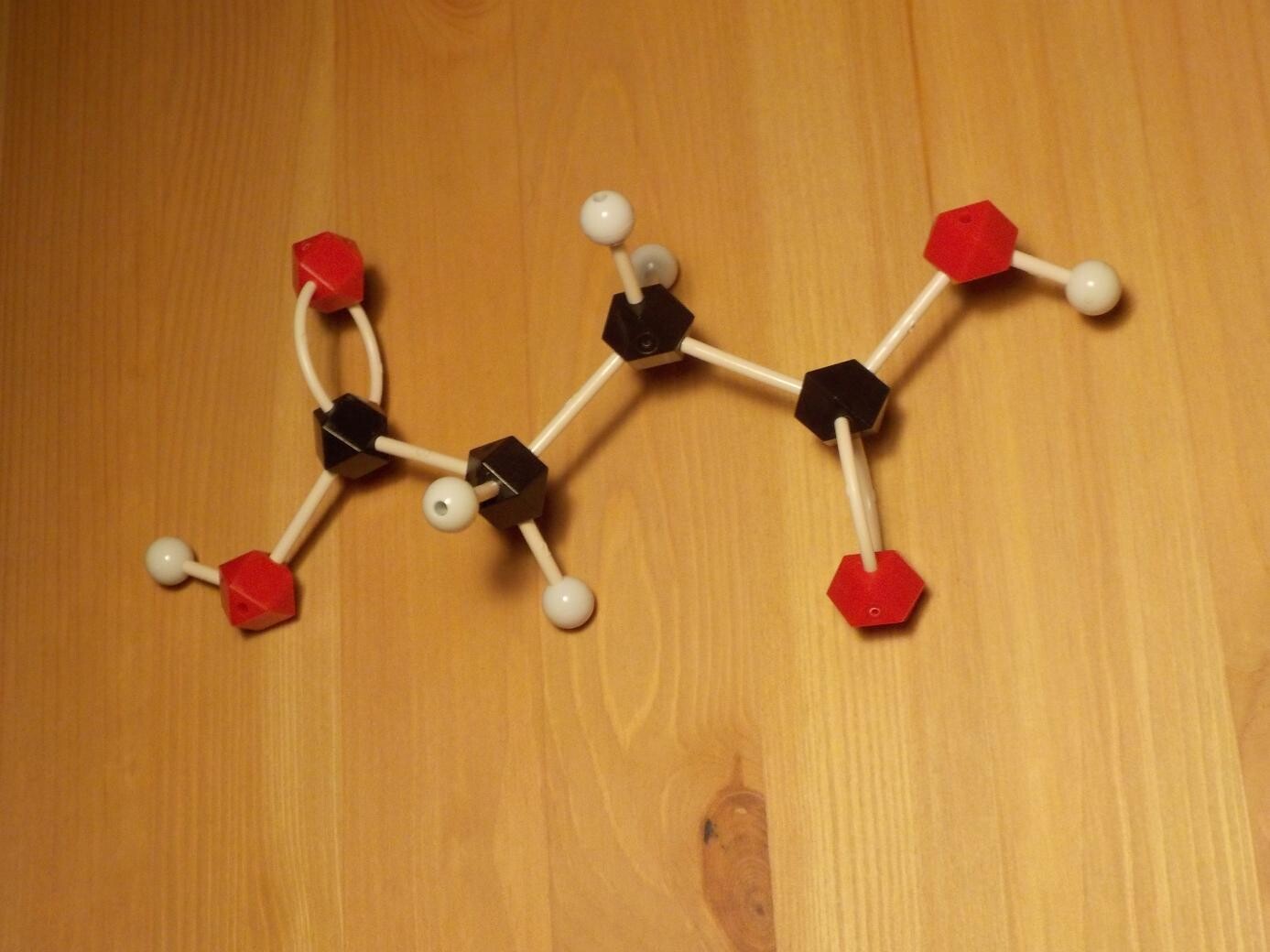
Succinic acid is not rare or obscure; it is a major industrial product with its leading applications (bearing in mind that flux is a tiny niche) in plastics manufacturing and as a food additive. It produces a flavour described in Wikipedia as somewhat sour and astringent to umami. Obtaining pure succinic acid in small quantities may not be easy for the home hobbyist, though, and in my formulation I opted to use citric acid instead.
Citric acid is also an extremely common activating ingredient in commercial water-wash fluxes. It has a chemically similar structure to succinic acid, with three organic acid groups and an alcohol (making it an "hydroxy acid") on a three-carbon backbone. It is found in citrus fruits, hence the name - it is what makes lemons sour - but most of the world's industrial production of citric acid is done by fermentation with specialized strains of moulds. The main use of citric acid is as a food and drink additive, for its sour flavour and pH-buffering ability.
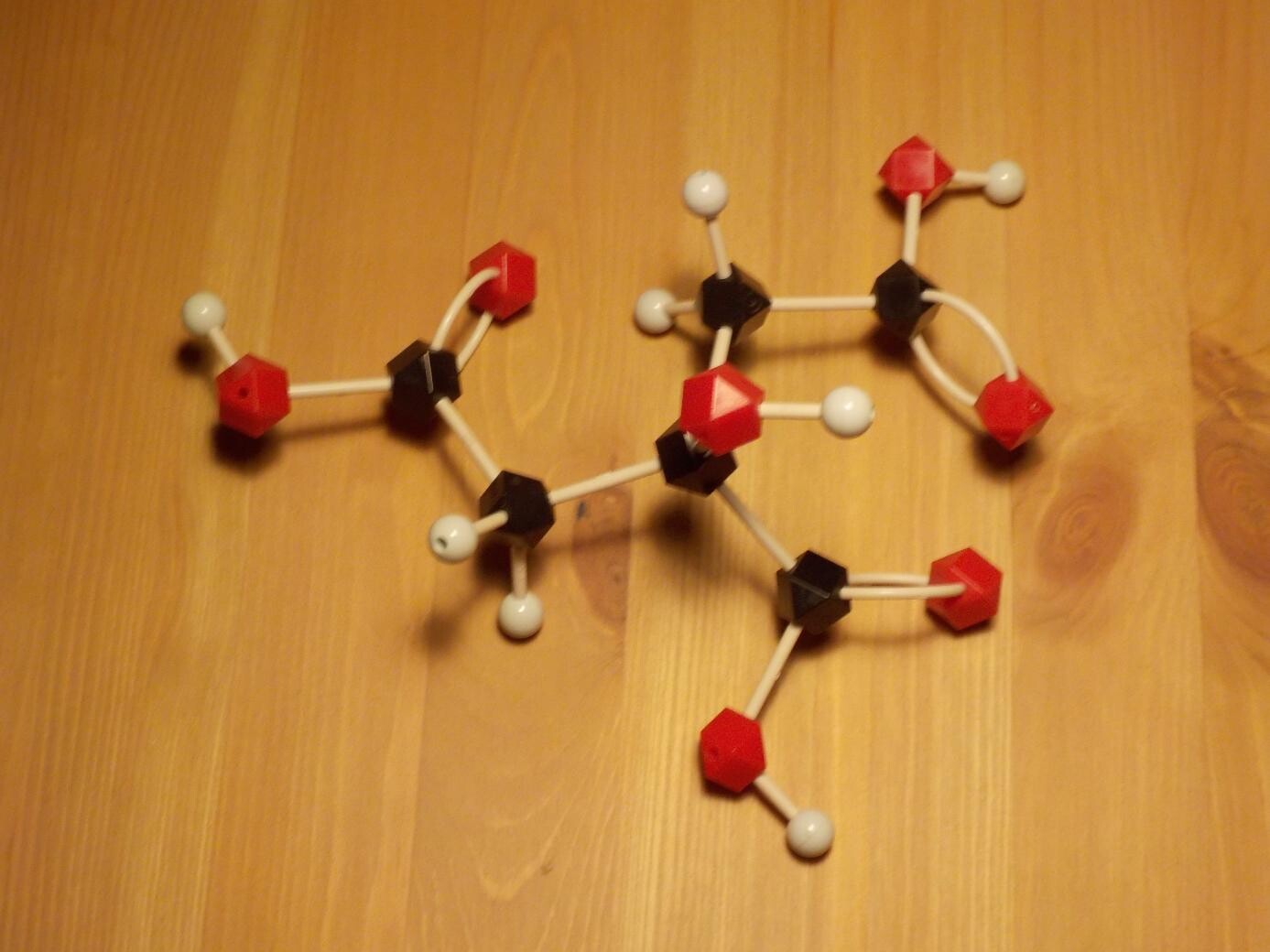
One of the nice things about citric acid is that it can serve as a chelating agent. In solution, the citric acid ion can wrap around a metal ion, keeping the metal soluble and reducing its tendency to participate in reactions. The space-filling model below illustrates the effect. Citric acid in foods can serve as a specialized preservative by chelating metals that would otherwise catalyse oxidation. In solder flux, this same effect means that citric acid can remove soluble metal ions that might participate in corrosion - but only when the flux residue is washed away.

In my formulation I also added glycerol, another popular ingredient in commercial fluxes. It serves multiple purposes. It's a high-boiling liquid that flows well when heated, so even though it does not solidify at room temperature, to some extent it functions as a resin, as described in the first article of this series. Glycerol is also a good, if slow, solvent for citric acid, so it helps keep the citric acid dissolved in the isopropyl alcohol that is the main solvent in my formulation. And at room temperature it thickens the solution, helping it stay on the surfaces where it's applied; and although isopropyl alcohol already has low surface tension, the glycerol may have some surfactant effect too.
Here's the complete formulation, with percentages by weight.
- Citric acid: 18%
- Glycerol: 10%
- Potassium sorbate: 0.2%
- Tap water: enough to dissolve the potassium sorbate (should be less than 1%)
- Isopropyl alcohol: to make 100% total
Dissolve the potassium sorbate in just enough water to fully dissolve it, then combine with the rest of the ingredients. The citric acid dissolves only very slowly, but it should all dissolve eventually. I stirred mine by hand 30 minutes with occasional breaks, and after that it was almost but not quite fully dissolved and I ran out of patience and put it in a jar as it was, with a little bit of undissolved citric acid in suspension; when I next checked a few hours later, it had completely dissolved. Some better procedure may be possible, such as heating it, or stirring it just enough to suspend the solids for transfer to a sealed container and then waiting for the solvent to do all the work. Increasing the percentage of glycerol might possibly help, too - glycerol is theoretically one of the best possible solvents for citric acid - but I didn't want to push that too far. If you have a magnetic stirrer, it might be helpful. Don't leave the mixture sitting uncovered for extended periods of time because the alcohol will evaporate.
I have not tried this, but if you wanted a "paste" flux, it would make sense to just leave out the isopropyl alcohol and mix up the other ingredients of this formulation in otherwise the same quantities. Probably not all of the citric acid would dissolve in the glycerol, but you'd end up with about the right pasty consistency and could vary it to taste by using more or less glycerol. Maybe for additional style points you could grind up the citric acid into a fine powder.
Here's my video of preparing this flux. The only hard part is the amount of stirring involved.
Even though it uses alcohol as the main solvent in the formulation, this is a water-wash flux. It should be compatible with all solder alloys, but because it is a very active flux and it leaves a corrosive residue that attracts water from the air, it should not be left on the joint after soldering. Clean it off (with tap or, preferably, distilled water; other common flux solvents may work too but may need extra quantities or scrubbing) within an hour of applying it, and do not use it on parts that are not water-washable, nor on stranded wire because of the difficulty of cleaning between the strands. All the ingredients except the isopropyl alcohol are routinely used as food additives, so the toxicity of this formulation is low, but you still shouldn't breathe the alcohol vapours produced when applying the flux, nor the smoke produced during soldering with this flux. It may be irritating if left on skin for an extended period of time; wash it from skin with water.
Citric acid is available from baking-ingredient suppliers, home brewing suppliers, and even some well-stocked grocery stores and pharmacies, sometimes under the name "sour salt" although it is not a salt at all. Its most common application is simply for making things taste sour. It's also commonly used in the home as a cleaning agent, especially for removing hard-water scale from tea and coffee pots. I bought mine from Canadian Home Brew Supplies because they had several ingredients I needed for this and other projects and it was convenient to get them all at once.
Potassium sorbate is available from home brewing suppliers, because it is used as a fermentation stopper in wine-making. I don't recommend substituting sorbic acid in this formulation because the higher water solubility of potassium sorbate is important for water washability. Do not confuse sorbate with ascorbate (vitamin C).
Most pharmacies sell glycerol (also called "glycerin" or "glycerine"); it's used as a skin moisturizer and lubricant, and will probably be shelved near the rubbing alcohol, Vaseline, castor oil, and similar products. You can also get it from home brewing suppliers, who sell it for adding "body" to wines and spirits.
I recommend 99% isopropyl alcohol for this formulation, for better evaporation and because it's more likely to be useful in other electronics-related applications, but it would probably work to use 91% here and that might even make dissolving the citric acid a little easier.
In this article I've talked a bit about water-wash fluxes for electronic soldering, including the reasons for using them, their disadvantages, and some of the most common ingredients in them. I've also presented my own formulation for a water-wash flux hobbyists can make at home. Please comment and share, and stay tuned for my next entry, in which I'll describe a modern no-clean flux.
◀ PREV Homemade traditional resin flux || DIY no-clean-style flux NEXT ▶

MSK 015 Quad VCA
US$447.24 including shipping
Comments
I don't know what would be causing the effects you describe, but a few thoughts are: don't leave out the glycerol, it may be necessary to get the liquid to spread out and stick. Don't use too high a percentage of citric acid; I used 18% and got good results but even that may really be high and it might be worth trying less. Patent 5,452,840 seems to be suggesting 20% to 40%, but that's in water (which may be different), and other solder-flux patents usually recommend smaller percentages of activators, like 1%-5%, in non-water solvents. Do solder soon after applying flux, and clean the board soon after soldering; it should be okay for like an hour or two, and certainly shouldn't need to be soldered within two seconds, but leaving the flux residue on for a long time will definitely cause corrosion. Use water for cleaning, other solvents (such as might be used for cleaning traditional rosin flux) may not easily dissolve citric acid or its residue. Do clean the soldering iron tip with water too, not only a metal coil, because it's subject to corrosion as well as the joint.
I doubt that your problems are just caused by dirty citric acid because most common sources of citric acid will be intended for food use, and they will be very clean for that reason, and I doubt there's much that could be a contaminant that would cause problems in flux use anyway. I suppose one possibility would be that if somehow your citric acid were mixed with *sugar* in particular - like if it were a powdered lemonade mix instead of just plain citric acid - then that could cause trouble. Sugar would melt and caramelize into a gooey brown residue under soldering conditions, but even that should be removable with water a little bit of scrubbing. Using alcohol as the solvent instead of water would probably help rule out sugar, because alcohol will not dissolve sugar very well.
If your joints really are cold, not just looking *like* cold joints for some other reason, then you have a temperature problem and that wouldn't be a flux issue.
Best of luck and I hope you'll share the resolution if you find one!
The citric acid i am using is food grade and it dissolved in alcohol slowly but it did dissolve, do note that i was using ethanol not isopropyl, i do not know if that makes any difference as isopropyl is really hard to get
The temperature was 290-300c on KSGER T12 soldering iron, also i am using leaded solder with rosin core but i doubt that changes much
I have tested both with water (store bought distilled water for irons and such) and ethanol (it was 70% as i didnt have any other at the time) with about 30% citric acid by weight
For next test batch i am also going to try using fluxless leaded solder to see pure results, i will be back with results in few days hopefully
The effect is A LOT better with 1 wt. % citric acid instead of 20-30% as the patent said, even with water on low temperatures (290 - 300c) it works
Water is pretty bad as the base as it splashes and ruins it on temperatures higher than 310c, glycerin actually works pretty well but pure glycerin fumes A LOT, even more than pure rosin
I am going to try this recipe too when i get my hands on alcohol and more glycerin, one more thing im thinking of trying is starch and vaseline as they are both pretty easy to get